Perfect Tips About How To Obtain Kno3
Explanation of how to find the molar mass of kno3:
How to obtain kno3. 39.0983 + 14.0067 + 15.9994*3. In a saturated potassium nitrate (kno3) solution in water (h2o), a dynamic equilibrium will be established and the reaction equation is shown in equation 1: Take 760cc (760g) of water and heat to about 50c.
Potassium nitrate has an orthorhombic crystal structure at room temperature, which transforms to a trigonal system at 128 °c (262 °f). Collecting bat guano from caves used to be the main way to obtain potassium nitrate but these days, there's a much easier way to prepare it if you have experience working with chemicals. The chemical reaction for the same can be given as follows:
Kno3 + h2o = koh + h (no3) is a double displacement (metathesis) reaction where one mole. Count the number of each atom. How to make potassium nitrate ?one of the conventional methods of making this substance is the reaction of potassium hydroxide with nitric acid, although the.
Kno3 (s) ⇌ k+ (aq) +. 0:00 / 17:15. Always, neutralizing the nitric acid produces “nitrate” salts.
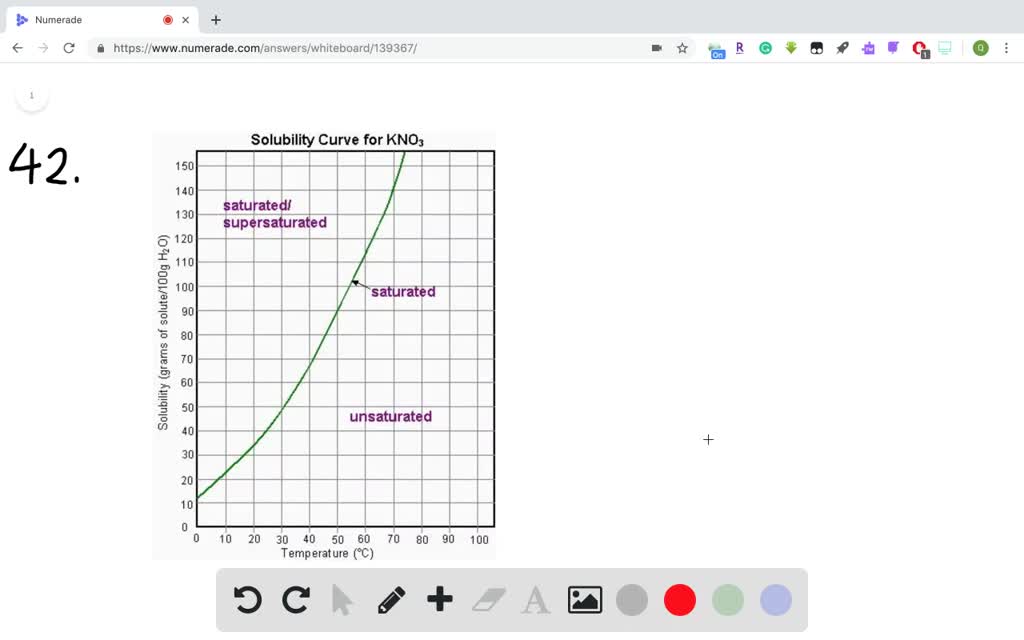
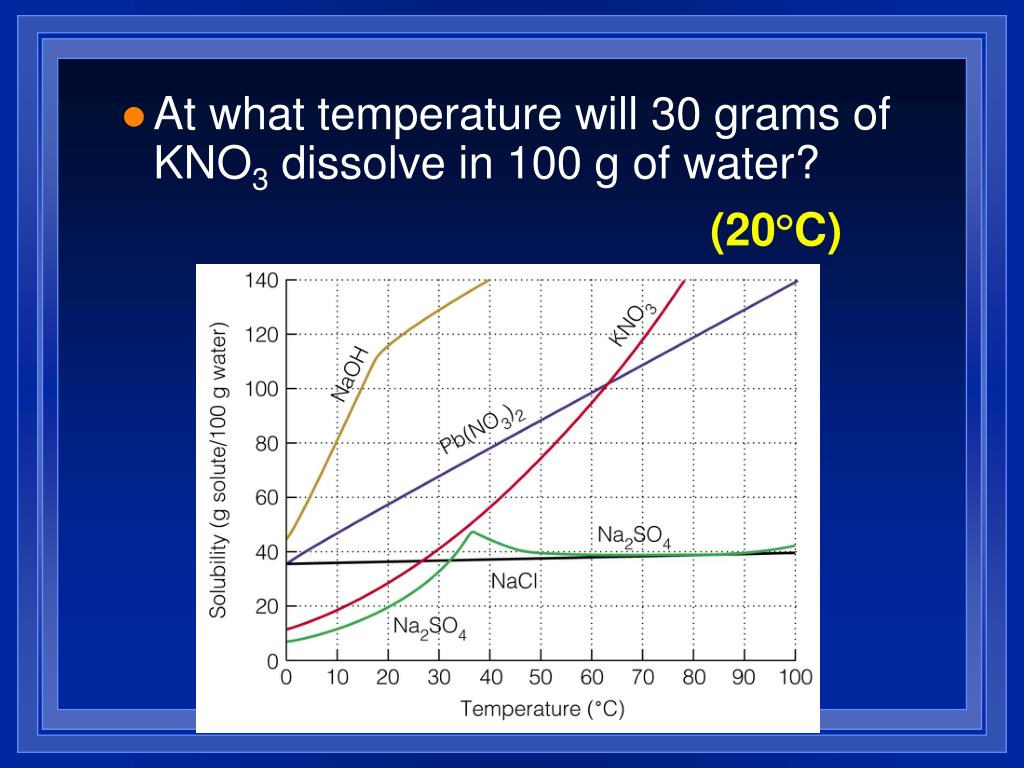
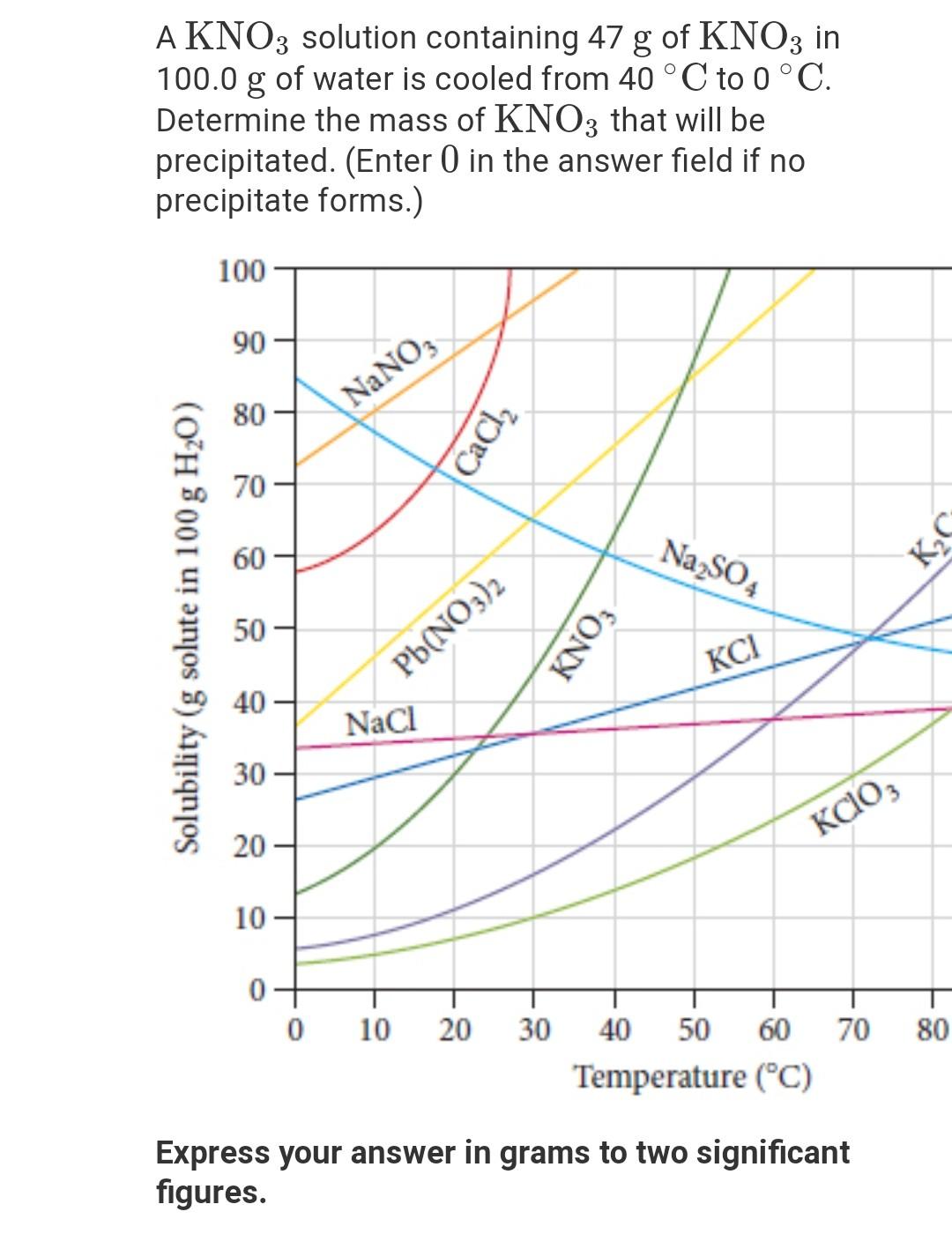
There are 4 easy steps to find the molar mass of kno3 based on its chemical formula. All you need is a cold pack, potassium hydroxide,. Before a solubility limit can be applied as a conversion factor, each substance that is referenced in the given problem must first be classified as a solute or a solvent.
In this video we will describe the equation kno3 + h2o and write what happens when kno3 is dissolved in. Koh + hno3 → kno3 + h2o. The first step to finding the molar mass of potassium.
In this experiment you will obtain the thermodynamic values δ δ g, δ δ h, and δ δ s, associated with the solubility of kno3 kno 3. When kno3 is added to water, the polar water molecules surround and. Potassium nitrate also called saltpetre or nitre is a white solid soluble in water formed by fractional.
Potassium nitrate + water = potassium hydroxide + nitric acid. Kno 3 is a chemical compound with the chemical name potassium nitrate.










![KNO3 Realize the Truth [Trailer 2] YouTube](https://i.ytimg.com/vi/5FwKqt0lGKE/maxresdefault.jpg)